Structural Biology of Membrane-Associated Photosynthetic Proteins
Unraveling the Molecular Mechanisms of Complex Membrane-Associated Photosynthetic Proteins
The growing global demand for energy presents a critical challenge, as most conventional energy sources are either finite, harmful to the environment, or major contributors to greenhouse gas emissions. Solar energy offers a clean and sustainable alternative, but current technologies for converting sunlight into transportable fuels remain inefficient and costly. Remarkably, nature has already solved this problem through photosynthesis—a process by which plants, cyanobacteria, and algae efficiently capture and utilize solar energy.
Our research aims to unravel the intricate molecular mechanisms behind photosynthesis. Using advanced spectroscopic techniques and cutting-edge structural analysis methods, we strive to decode these natural processes at the atomic level. This foundational knowledge not only deepens our understanding of biological energy conversion but also provides a blueprint for developing artificial photosynthesis technologies, paving the way for sustainable energy solutions.
Our lab specializes in exploring the structural and functional mechanisms of complex membrane proteins central to photosynthesis. At the heart of our research is Photosystem II (PSII)—a key model system for studying membrane protein complexes and their role in life-sustaining processes. By utilizing state-of-the-art tools such as X-ray free electron laser (XFEL) crystallography, cryo-electron microscopy (cryo-EM), and a range of spectroscopic techniques, we capture dynamic biological processes at near-atomic resolution. Through collaborative efforts with leading national and international research groups, we address significant challenges in structural biology and photosynthesis research.
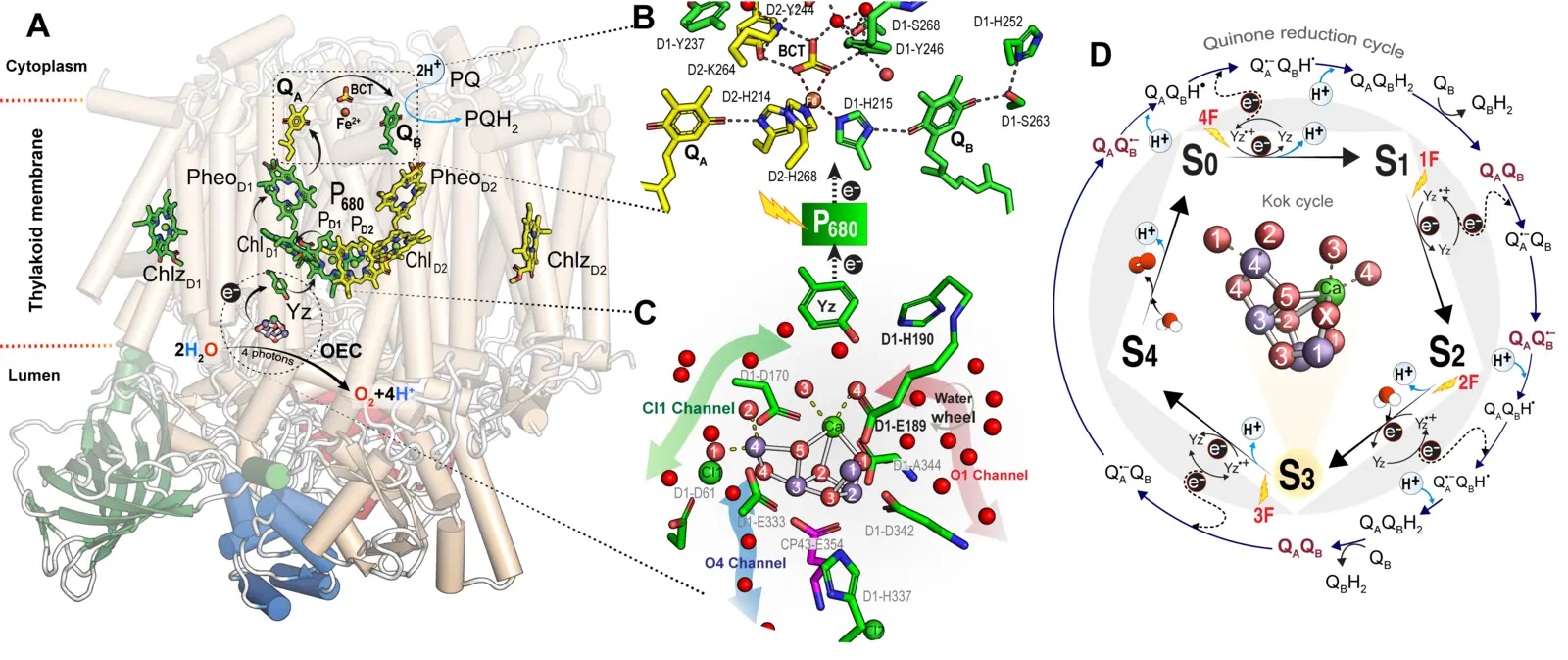
Structure of monomeric PSII structure, showing cofactors involved in light-induced charge separation and stabilization through electron transfer. A) Highlights the monomeric PSII structure, showing cofactors involved in light-induced charge separation and stabilization through electron transfer. (B) Provides a close-up of the acceptor site with plastoquinones QA and QB. (C) Explains the Mn₄CaO₅ cluster and its three channels (O1, O4, Cl1). (D) Illustrates the Kok cycle for water oxidation, detailing the sequential oxidation states (S₀–S₄) of the Mn₄CaO₅ cluster, driven by light flashes, and its role in quinone reduction. Key structural components, cofactors, and electron/proton transfer pathways are visualized. (Hussein et al. Science 2024)
Why Photosystem II (PSII)?
PSII is the centerpiece of our research due to its vital role in the light-driven splitting of water into oxygen, protons, and electrons—a process fundamental to sustaining Earth’s oxygen cycle and all aerobic life. This unique biological complex provides an unparalleled system for investigating key biochemical phenomena, including:
- Coupled Catalysis: PSII integrates two reaction sites for water oxidation and plastoquinone reduction, offering insights into the tightly orchestrated biochemical reactions within a single protein complex.
- Oxygen-Evolving Complex (OEC): At the core of PSII, the unique Mn₄CaO₅ cluster serves as a catalytic hub for water splitting. Its study sheds light on metal ion dynamics and enzymatic processes essential for oxygen evolution.
Our work pushes the boundaries of bioenergetics, enzymatic research, and membrane protein structural biology, driving innovative discoveries that bridge fundamental science and practical applications in energy and sustainability.



