Structural and Mechanistic Basis of ABC Transporters
ATP-binding cassette (ABC) transporters are transmembrane proteins found in all forms of life. They utilize ATP to ferry a multitude of different substrate cargos across cellular membranes and thereby assume diverse physiological functions. Consequently, malfunctioning ABC transporters are the root cause of several human diseases, including cystic fibrosis and hypercholesterolemia. Furthermore, ABC transporters contribute to multidrug resistance (MDR) in human pathogens and cancer cells. Significant insights into the activity of ABC transporters have been gained from functional and structural studies in recent years, and several mechanistic models have been proposed. However, fundamental questions concerning their mode of operation in substrate binding and translocation remain.
Our group is focusing on ABC transporters that translocate peptides. In particular, we are interested in ABC systems involved in shaping the adaptive immune response. These systems include the heterodimeric human transporter associated with antigen processing (TAP1/2) and lysosomal TAP-like (TAPL). TAP1/2 is embedded in the membrane of the endoplasmic reticulum (ER) and constitutes the central component of the supramolecular peptide-loading complex (PLC). It shuttles cytosolic peptides into the ER where they are loaded onto major histocompatibility complex class I (MHC I) molecules for presentation on the cell surface. The presented peptides allow components of the immune system to detect virally infected and aberrantly transformed cells.
In addition to investigating TAP1/2 and TAPL directly, we employ the bacterial protein TmrAB, a functional homolog of TAP1/2, as model system for the human transporters. We applied single-particle cryogenic electron microscopy (cryo-EM) and X-ray crystallography to characterize the asymmetric nature and substrate-binding cavity of TmrAB. Moreover, cryo-EM experiments under turnover conditions enabled us to outline the full functional cycle and conformational spectrum of an ABC transporter in lipid environment. These structural approaches, which our lab combines with biochemical and biophysical studies of transporters reconstituted in nanodiscs or liposomes, will allow us to deepn our understanding of these fascinating molecular machines in future analyses.
The Lysosomal Polypeptide Transporter TAPL
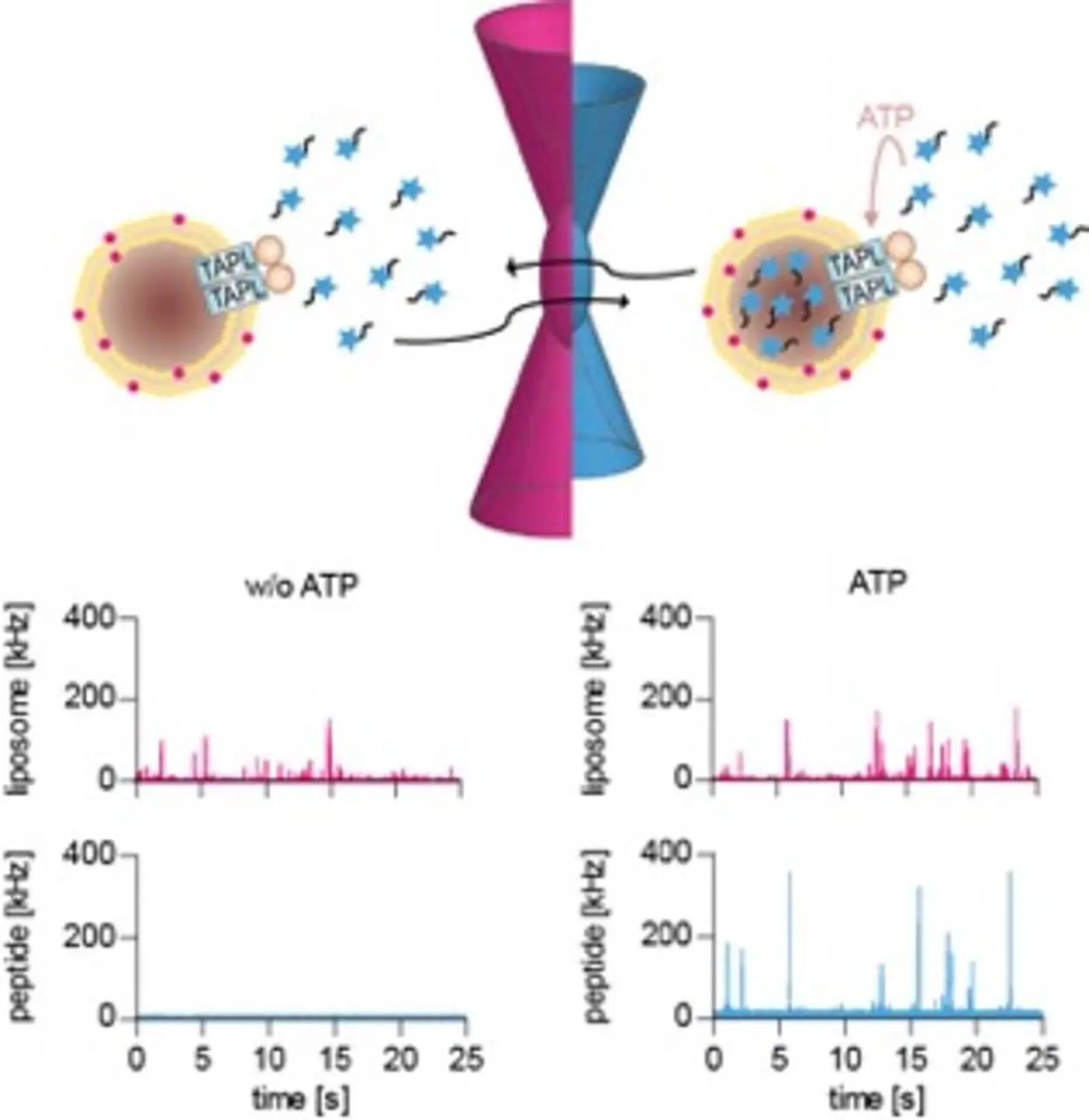
TAPL is a homodimeric lysosomal peptide transporter. We are interested in the intracellular trafficking of this transporter in respect of the subcellular route as well in the machinery involved in this process. The physiological function of TAPL in lysosomes is still enigmatic, however, it appears to be associated with biogenesis of this organell. In the past, we have developed the functional reconstitution of TAPL in proteoliposomes and methods to follow the peptide transport on single transporter level. With these tools in hand, we are able to decipher details of the transport mechanism. Recently, we could demonstrate that ATP hydrolysis is uncoupled from peptide transport and that the efficiency of GTP is less than ATP in respect of transport.
Publications:
Thomas C, Tampé R
Structural and mechanistic principles of ABC transporters.
2020, Annu Rev Biochem, 89,605-36; doi: 10.1146/annurev-biochem-011520-105201
Stefan E, Hofmann S, Tampé R
A single power stroke by ATP binding drives substrate translocation in a heterodimeric ABC transporter.
2020, eLife, 9,e55943; doi: 10.7554/eLife.55943
Hofmann S, Januliene D, Mehdipour AR, Thomas C, Stefan E, Brüchert S, Kuhn BT, Geertsma ER, Hummer G, Tampé R, Moeller A
Conformation space of a heterodimeric ABC transporter under turnover conditions.
2019, Nature; doi:10.1038/s41586-019-1391-0
Bock C, Zollmann T, Lindt KA, Tampé R, Abele R
Peptide translocation by the lysosomal ABC transporter TAPL is regulated by coupling efficiency and activation energy.
2019, Sci Rep, 9,1184; doi: 10.1038/s41598-019-48343-6
Thomas C, Tampé R
Multifaceted structures and mechanisms of ABC transport systems in health and disease.
2018, Curr Opin Struct Biol, 51,116-28; doi: 10.1016/j.sbi.2018.03.016
Nöll A, Thomas C, Herbring V, Zollmann T, Barth K, Mehdipour AR, Tomasiak TM, Brüchert S, Joseph B, Abele R, Oliéric V, Wang M, Diederichs K, Hummer G, Stroud RM, Pos KM, Tampé R.
Crystal structure and mechanistic basis of a functional homolog of the antigen transporter TAP.
Proc Natl Acad Sci U S A. 2017 Jan 24;114(4):E438-E447. doi: 10.1073/pnas.1620009114. Epub 2017 Jan 9.
Lehnert E, Mao J, Mehdipour AR, Hummer G, Abele R, Glaubitz C, Tampé R.
Antigenic peptide recognition on the human ABC transporter TAP resolved by DNP-enhanced solid-state NMR spectroscopy.
J Am Chem Soc. 2016 Oct 26;138(42):13967-13974. doi: 10.1021/jacs.6b07426. Epub 2016 Oct 17.
Bechara C, Nöll A, Morgner N, Degiacomi MT, Tampé R, Robinson CV
A subset of annular lipids is linked to the flippase activity of an ABC transporter.
2015, Nat Chem, 7,255-62; doi: 10.1038/nchem.2172
Kim J, Wu S, Tomasiak TM, Mergel C, Winter MB, Stiller SB, Robles-Colmanares Y, Stroud RM, Tampé R, Craik CS, Cheng Y.
Subnanometre-resolution electron cryomicroscopy structure of a heterodimeric ABC exporter.
Nature. 2015 Jan 15;517(7534):396-400. doi: 10.1038/nature13872. Epub 2014 Nov 2.
Zollmann T, Moiset G, Tumulka F, Tampé R, Poolman B, Abele R
Single liposome analysis of peptide translocation by the ABC transporter TAPL.
2015, Proc Natl Acad Sci USA, 112,2046-51; doi: 10.1073/pnas.1418100112
Project members
PD Dr. Rupert Abele
Group Leader
phone +49-(0)69-798-29437
abele@em.uni-frankfurt.de
Matija Pecak
PhD Student
phone: +49-(0)-69-798-29471
pecak@em.uni-frankfurt.de
Christoph Nocker
PhD Student
phone: +49-(0)-69-798-29471
nocker@em.uni-frankfurt.de